In the United States, the Fda (FDA) serves as a main entity setting requirements for compliance in pharma. In the EU, the European Medicines Firm (EMA) watches on drug quality. These and numerous other companies around the world ensure to promote public health by assessing, controling, and monitoring all things pharma.
The list below aspects, to name a few, are triggering regulative bodies to present pharmaceutical compliance programs:
- The growing pressure on the health care and pharmaceutical markets to guarantee client security and safeguard public health
- The increasing quantity of fake items on the marketplace
- Rising drug advancement, screening, and circulation expenses
- The requirement for helping with compliance in pharmaceutical market with suitable laws
- The requirement for preserving constant quality requirements throughout various areas and markets.
Compliance programs intend to promote openness, security, and effectiveness in the market. Nevertheless, accomplishing compliance in pharma can be complicated and pricey.
Embracing pharmaceutical innovation services, business can get an important ally in their efforts to accomplish and keep compliance. Listed below, we share our experience crafting pharma and life science software application and emphasize essential methods which innovation enhances pharmaceutical compliance.
4 methods which innovation enhances compliance in pharma
1. Getting rid of silos
Cloud-based information management, information warehousing, huge information analytics, and other innovations can assist pharma business combine information that was formerly saved throughout siloed systems.
Consolidated information storage enables pharmaceutical business to get a 360-degree view of their information and discover compliance problems early on.
One example of a business leveraging contemporary tech to enhance pharma compliance by improving information management is Pfizer. The leading pharma corporation counts on an electronic file management system that combines scientific trial information from several sources for a single, unified view. It easily tracks the development of scientific trials, recognizes patterns and patterns, and guarantees that trial information is precise and current.
Another example originates from Johnson & & Johnson, which utilizes a cloud-based information management system that incorporates all business information originating from various sources. The service helps with the business’s efforts in accomplishing compliance in pharma by preserving precise and current records of all activities associated with drug advancement, screening, and circulation. This consists of tracking all phases of drug advancement, consisting of research study, scientific trials, regulative approval, and post-market monitoring.
2. Allowing innovative reporting
By counting on new-gen reporting tools, pharmaceutical business can now produce personalized reports of any intricacy (in genuine time also). This assists them get much deeper insight into their information, recognize possible pharma compliance threats, and take proactive steps to resolve them.
Furthermore, innovation tools can assist pharma business prevent replicate or inconsistent reports throughout various departments or areas, making sure that the information sent to regulative bodies corresponds and precise
The Jonhson & & Johnson’s information management system discussed above is a worthwhile example of how innovation helps with pharma compliance through effective negative occasion reporting. The Johnson & & Johnson’s service enables the business to effectively track and report negative occasions and negative effects, making sure that all essential details is gathered and sent to regulative bodies in a prompt way.
Another international pharmaceutical business, Sanofi, utilizes advanced reporting to facilitate their efforts in accomplishing compliance in pharma. Innovation tools permit the business to produce adjustable reports that satisfy the particular requirements of each regulative body they handle. All reports are produced instantly, conserving time and decreasing the capacity for human mistake.
3. Assisting in service procedures
Another usage case of innovation in helping with pharma compliance is enhancing service procedures. Particularly, innovation can assist improve and even automate the following jobs:
- Administration, tax, and financing: accounts payable, payment processing, bank reconciliation, and tax reporting
- Buying: cost analysis, cost report management, procurement information management, provider relationship management, and agreement management
- Supply chain: need forecasting, stock and possessions management
- Personnels: travel and cost management, staff member information management, time record recognition, and others.
Lots of pharma business around the world are leveraging the power of tech to assist in and automate ordinary workflows For instance, Novo Nordisk, an international pharmaceutical business headquartered in Denmark, utilizes innovation to automate indirect tax compliance management throughout more than 50 nations the business runs in, which substantially enhances their pharma compliance management programs.
The service is incorporated with the business’s ERP system, which assists rapidly recognize the possible locations of non-compliance. It likewise offers real-time presence into the business’s tax position, making it possible for Novo Nordisk’s executives to make more educated choices and enhance their tax technique.
Another example of counting on service procedure optimization for preserving compliance in pharma originates from AstraZeneca. The international pharmaceutical business has actually embraced a supplier management system that enables them to successfully handle their international supplier relationships, display supplier efficiency, and watch on the suppliers’ compliance with regulative requirements. The system keeps a main database of suppliers and their associated compliance documents, consisting of credentials, accreditations, and monetary records.
4. Accelerating drug advancement
The growing usage of innovation assists pharma business decrease the time and expense connected with bringing brand-new drugs to the marketplace.
The methods which contemporary tech enables accelerating drug advancement – – while assisting keep pharma compliance – – are numerous:
- Advanced information analytics and artificial intelligence tools can speed up drug discovery and recognition of possible drug prospects
- Utilizing computer system simulations and digital twins can assist anticipate drug habits prior to it is evaluated live
- Electronic health records and other client information management systems can assist recognize qualified clients for scientific trials and accelerate recruitment
- Wearable gadgets and keeping an eye on innovation can supply real-time information on clients in scientific trials, lowering the time and expense of gathering information
- Web of Things-powered production devices can speed up the production of drug models while protecting drug quality
- Robotic procedure automation (RPA) tools can automate recurring jobs in drug advancement, maximizing scientists’ time
- Cloud computing innovations can supply scalable and affordable storage and processing of big quantities of information, offering faster analysis and decision-making.
One example of a business that has actually currently found the worth of tech in accelerating drug advancement is GSK. The British pharmaceutical and biotechnology business utilizes expert system to evaluate drug substances quicker, effectively, and properly. The business likewise counts on AI to recognize possible drug prospects, substantially lowering the time it requires to move them to scientific trials.
Another example of a business utilizing tech to enhance pharma compliance originates from Novartis. The Swiss pharma corporation utilizes AI to examine large quantities of information from its drug discovery and advancement programs. Particularly, they use AI to examine client information, preclinical information, and hereditary details to recognize possible drug targets and enhance drug prospects.
Novartis likewise utilizes AI to create brand-new drug substances with particular residential or commercial properties and to anticipate how those substances will engage with biological targets
Barriers to utilizing innovation for pharma compliance management
While innovation can be exceptionally useful in accomplishing compliance in pharma, there are a number of barriers that business should conquer in order to use the amount of tech.
Among the most substantial ones is the absence of digital maturity within the sector. Digital maturity describes the level to which a company is counting on digital tools and innovation. Lots of pharma business still depend upon tradition systems, which can be hard to incorporate with contemporary innovation services.
A study carried out by DT onsulting amongst international pharma management highlights the following factors for the low digital maturity in the sector:
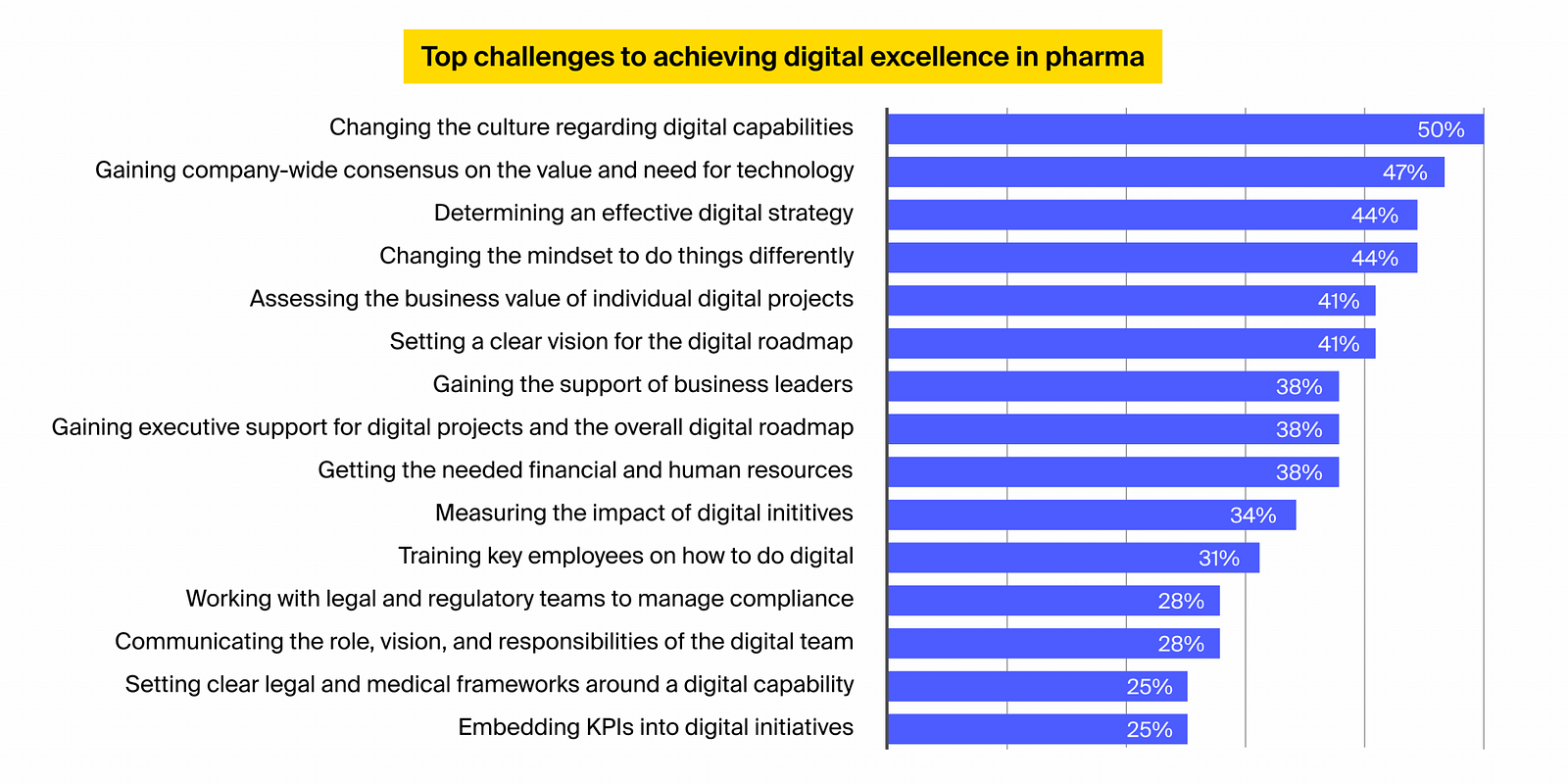
Improving the sector’s digital maturity and improving pharma compliance efforts requires sector-wide modification and needs dealing with the following traffic jams:
- The viewed threat connected with embracing brand-new innovation
The pharma market is extremely controlled, and any modification to procedures or systems should be thoroughly assessed and evaluated to guarantee compliance with regulative requirements. This mindful method can cause slow decision-making, leading to hold-ups in the adoption of brand-new innovation.
Option: To decrease the threats connected with embracing innovation services, we recommend you to team up with regulative authorities and engage with them early in the change procedure in order to guarantee pharmaceutical compliance and get a buy-in.
Another service to think about is beginning your digital change effort with a pilot research study. Carried out in a regulated environment, it might assist you evaluate brand-new innovations, along with show their compliance and security.
- The absence of innovation standardization in the sector
Lots of pharma business have actually grown through mergers and acquisitions, leading to a patchwork of tradition systems and procedures that are typically hard to incorporate. This can make it challenging to carry out brand-new innovation services, as they might not work with the existing systems.
Option: To conquer this problem, we suggest running an extensive evaluation of existing systems and procedures to recognize possible traffic jams, focusing on the application of innovation services that work with existing systems, and turning to iterative application to guarantee smooth combination of all innovation elements.
- The absence of interoperability
The pharma sector is extremely based on information. Information silos and the absence of interoperability can prevent the adoption of innovation and present a difficulty to preserving compliance in pharma. Various departments within a pharma business might utilize various systems and even various variations of the very same system, making it challenging to combine information and get a holistic view of the company’s operations.
Option: To guarantee information stability and software application interoperability, think about establishing an extensive information technique that would specify information governance, ownership, and standardization policies. It likewise settles to perform routine information audits to recognize information silos and disparities.
- The absence of digital abilities and know-how within the market
The pharma sector has actually typically been sluggish to accept brand-new innovation and might do not have the essential resources, abilities, and know-how to carry out digital services.
Option: To close the know-how space, pharmaceutical business can either buy hiring skilled experts who can lead the application of the brand-new innovation or rely on external experts with the know-how establishing services for pharma to supply tactical and innovation management.
On a last note
Although traditionally, the pharmaceutical sector has actually been rather sluggish in embracing innovation for much better pharma compliance management, the outlook is altering. Regulative authorities themselves trigger the market to get on the development bandwagon.
In the United States, the FDA developed the emerging innovation program that motivates ingenious methods to pharmaceutical item style and production. Likewise, the Process Analytical Technologies (PAT) group developed by the EMA and the MHRA (Medicines and Health care items Regulatory Firm) leads development in the EU and UK.
The success stories of early adopters show: innovation services do make it simpler to accomplish and keep compliance in pharma. Still, to profit, it is vital to approach the application of unique services very carefully and with the possible difficulties in mind.To find out more about how innovation services can assist your business accomplish and keep compliance, contact our innovation experts.
Trying to find methods to improve pharma compliance? Contact ITRex for assistance.
Initially released here
The post How to utilize unique innovation to accomplish compliance in pharma appeared initially on Datafloq